IFX SC offers consistent steady-state levels1
Theoretical PK profiles of IFX IV (CT-P13) and IFX SC (CT-P13)
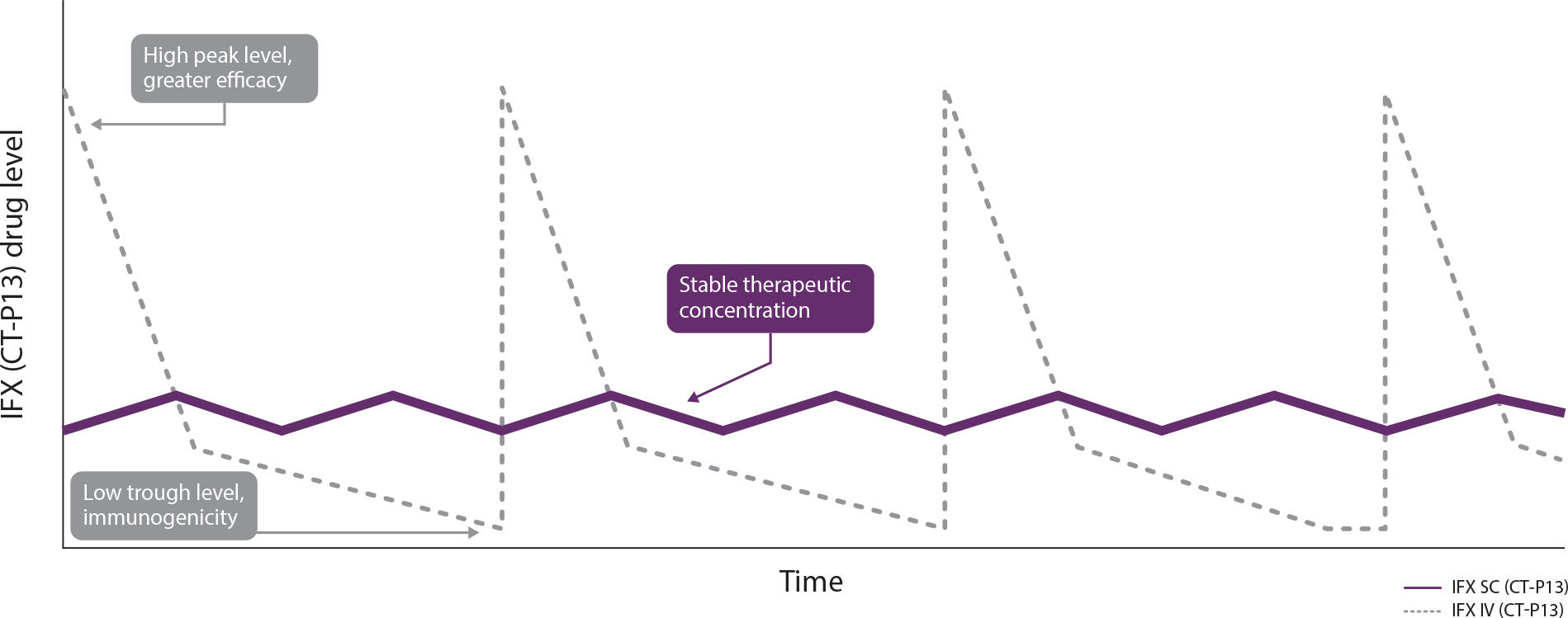
Chart adapted from Little RD, et al. J. Clin Med. 2022;11(20):6173.1
Study Note: This chart reflects hypothetical serum drug levels for illustrative purposes. The chart is derived from a comprehensive narrative review by Little et al.,1 which synthesizes findings from multiple studies. The review predominantly focuses on studies involving CT-P13, administered both IV and SC.
Current evidence does not establish a direct correlation between the pharmacokinetic attributes and clinical efficacy. The prognostic relevance of serum drug concentrations should be interpreted within the complete clinical context, not as the sole predictor of therapeutic outcomes.
LIBERTY UC Trial
ZYMFENTRA maintained steady and stable IFX trough levels in patients with UC2,3
- Post-Week 10, serum concentrations in the ZYMFENTRA™ group rose until Week 14 and stayed stable through Week 54 due to biweekly dosing
- During the SC maintenance phase, mean range (CV%) of observed trough was 14.6 to 16.3 μg/mL in the ZYMFENTRA group
– This is well above the minimum therapeutic threshold of 3 μg/mL and above the >7 μg/mL associated with mucosal healing
Mean IFX serum concentration in patients with UC
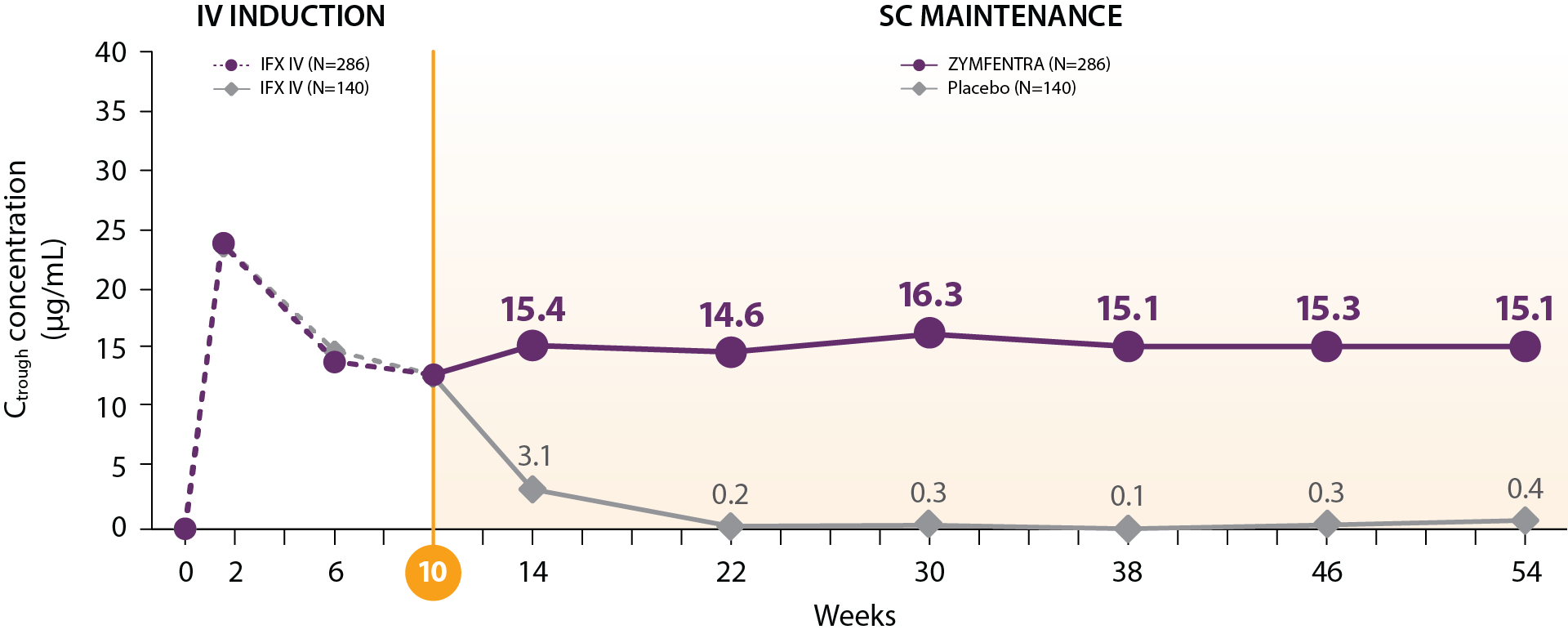
Study Note: From Week 0 through Week 10, patients were in the induction phase with IFX IV. In the treatment group, post-Week 10, patients were given ZYMFENTRA for maintenance. During Week 22 through Week 54, dose adjustments were allowed for patients who initially responded but then lost response according to the loss of response criteria.
Current evidence does not establish a direct correlation between the pharmacokinetic attributes of ZYMFENTRA and its clinical efficacy. The prognostic relevance of serum drug concentrations should be interpreted within the complete clinical context, not as the sole predictor of therapeutic outcomes.
LIBERTY CD Trial
ZYMFENTRA maintained steady and stable IFX trough levels in patients with CD2,3
- Post-Week 10, serum concentrations in the ZYMFENTRA group rose until Week 14 and stayed stable through Week 54 due to biweekly dosing
- During the SC maintenance phase, mean range (CV%) of observed trough was 13.3 to 14.8 μg/mL in the ZYMFENTRA group
– This is well above the minimum therapeutic threshold of 3 μg/mL and above the >7 μg/mL associated with mucosal healing
Mean IFX serum concentration in patients with CD
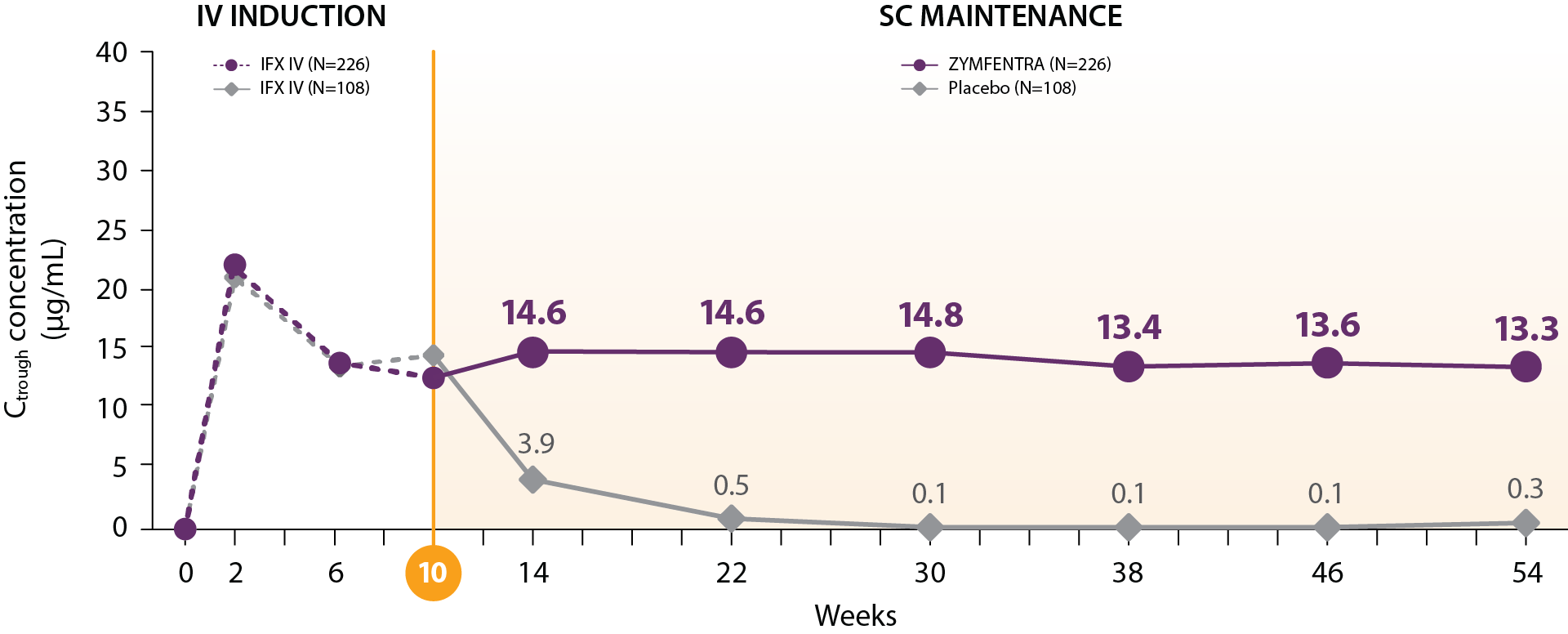
Study Note: From Week 0 through Week 10, patients were in the induction phase with IFX IV. In the treatment group, post-Week 10, patients were given ZYMFENTRA for maintenance. During Week 22 through Week 54, dose adjustments were allowed for patients who initially responded but then lost response according to the loss of response criteria.
Current evidence does not establish a direct correlation between the pharmacokinetic attributes of ZYMFENTRA and its clinical efficacy. The prognostic relevance of serum drug concentrations should be interpreted within the complete clinical context, not as the sole predictor of therapeutic outcomes.
CD=Crohn’s disease; CV%=coefficient of variation (standard deviation as a percentage of the mean); IFX=infliximab; IV=intravenous; SC=subcutaneous; UC=ulcerative colitis
References: 1. Little RD, Ward MG, Wright E, et al. Therapeutic drug monitoring of subcutaneous infliximab in inflammatory bowel disease—understanding pharmacokinetics and exposure response relationships in a new era of subcutaneous biologics. J Clin Med. 2022;11(20):6173.
2. ZYMFENTRA™ Prescribing Information, Celltrion, Inc. 2024.
3. Data on file, Celltrion, Inc.